Schedule: National Plan for R & D and innovation II Partnerships in priority areas
Research direction: D2-Energy
Competition: June 2007
Project no.: 21 037
Duration: September 2007 – Sept. 2010
Title: SUSTAINABLE ENERGY TECHNOLOGY, to promote indigenous uranium Mineral Resources, by optimizing the process for purifying uranium – TEDPUR
Contracting Authority: National Centre for Project Management – CNMP
Project Coordinator: National Institute for Metals Research and Development
ICPMRR Radioactive Resources, Bucharest, Romania
Project Director : Eng Antoneta Filcenco – Olteanu
Partner 1: Polytechnic University of Bucharest – Ecometalurgical Research and Expertise Center
Tel / Fax: 021 316 95 64, Tel. 402 95 92; E-mail: ECOMET[Ed]ecomet.pub.ro
Responsible project: Dr. Denisa Ileana Nistor
Partner 2: UBC Bacau University, Romania
Tel. 0745302970, Fax: 023451096, Eemail: denisanistor[Ed]yahoo.com,
Project Manager: Prof. Dr. Mircea Palamaru
Partner 3: ECO SC ECOPROIECT Ltd., Romania
Tel. : 021/ 3322992, Fax: 021/3322810, Email: cristina.niculescu[Ed]caloris.ro
Project Manager: fiz. Cristina Niculescu
The purpose of this complex project it is developing in partnership, a purification technologies of uranium by liquid–liquid extraction. The major objective of the project is increasing the efficiency of uranium purification in liquid-liquid extraction operation, by reducing the incidence of which lead to the formation of stable emulsions.
The project will reduce pollution and radioactive contamination by organic substances to the environment by reducing waste purification technology of uranium operation, consisting of stable emulsions.
Project’s contribution to the development of knowledge in the field Technological capacity is to increase and promote knowledge transfer in the field of manufacturing nuclear fuel, in order to optimize the technology of purification of uranium, with favorable consequences of increased processing efficiency and sustainable development.
Through its activities in the project, we aim to resolve issues relating to: reducing the formation of stable emulsions in uranium purification by liquid-liquid extraction by reducing the amount of impurities in the two phases, the supply of liquid-liquid extraction operation, reducing the possibility of formation of germs emulsifying process operating. Optimizing the operation of purifying uranium by liquid-liquid extraction generally results in loss reduction technology and uranium as a consequence increase the efficiency of processing operations by 5%.
Complexity project is to study the multitude of factors whose incidence contribute to stable emulsion through losses occurring uranium in waste technology, namely:
Ø acidic aqueous solutions uranium reduction below 2.8 N HNO3;
Ø temperature reduction solutions in cold periods than 20aC
Ø impurities in the aqueous phase;
Ø Impurities in the organic phase regenerated – degradation of TBF’s (mono and dibutyl phosphate).
Impact estimated to obtain the organic nature of the project is by reducing radioactive contamination by organic substances and pollution of the environment by reducing waste purification technology of uranium operation, consisting of stable emulsions.
Specific objectives:
Phase I – Done – deadline 12/15/2007
Documentation and technological solutions to optimize operation statement purification of uranium
Ø Study and analysis of solutions for optimizing the purification of uranium by liquid-liquid extraction
Ø Study on influence of impurities from the aqueous phase to form stable emulsions default losses of uranium to purification
Ø Study on influence of impurities from the organic phase in the formation of stable emulsions;
Ø Study on election of laboratory facilities for purifying uranium by liquid-liquid extraction-reextraction.
Phase Abstract
Third phase formation in uranium purification solvent lead to significant losses of solvent and other effects such as:
Ø loss of useful elements,
Ø decrease processing capacity installations;
Ø decrease of uranium separation efficiency;
Ø Decrease of purification rate of uranium impurities.
Reducing losses of uranium purification operation by reducing the incidence of which leads to the formation of stable emulsions is the overall objective of the project.
In the first stage, a documentary study the assertion of technological solutions to optimize uranium purification by liquid-liquid extraction; the book is divided into several sections according to the project implementation plan.
Formation of stable emulsion solvent extraction of uranium is due: aqueous uranium impurities, degradation of organic extractant and mode of operation.
Uranium impurities in aqueous solution from the technical concentrate which supplies uranium purification plant. Technological solution to prevent the appearance of impurities consists of product specifications for compliance sodium diuranat and impure uranyl nitrate.
SO (INCDMRR-ICPMRR – Bucharest) with P1 (PUB – CCEEM) conducted a study on the influence of impurities from the aqueous phase to form stable emulsions default loss of uranium from uranium purification, showing that the organic substances (humic acids, phenols, carboxylic acids, etc.), metallic compounds, particularly molybdenum and lead particulates apartments of the third phase.
University of Bacau team was primarily aimed to study the influence of impurities in organic phase to form stable emulsions. Also been studied and how diluent’s nature tributhyphosphate influence solubility.
SO (INCDMRR-ICPMRR – Bucharest) together with P3 (ECOPROIECT) made a documentary study on the appropriate equipment to purify uranium by liquid-liquid extraction-reextraction, showing that the nuclear industry specific settler blender, pulsed columns and columns extractors centrifuge. When choosing equipment must take into account several factors such as nature of solvents, flow, how to contact and mixing of phases, material which is made extractor, that working with radioactive solutions and more.
Variety of equipment on the market making it difficult to choose particular extraction equipment for a given separation.
Even if the choice involves a great deal of professional experience but the final decision should be based on a comprehensive economic calculation.
Phase II -Done – deadline 11/15/2008
Design and technology experimental model laboratory for studying uranium purification by liquid-liquid extraction
Specific objectives:
Ø Modeling uranium purification by liquid-liquid extraction and purification yield determination in conditions to achieve optimum process parameters
Ø Study on extraction equipment installation / reextraction with automatic control equipment and correct acidity
Ø Research on formation of stable emulsions in liquid-liquid extraction under the influence of high content of impurities in the aqueous phase
Ø Dissemination of information and participation in scientific meetings with project-specific communications
Ongoing stage of phase:
Ø Model solutions were prepared of impure uranyl nitrate (AUI) by dissolving sodium diuranat technical in nitric acid;
Ø Model solutions were characterized in terms of uranium content and impurities (particulates, acid free, Cl–, Fe, Mo, Na+SO42 –);
Ø Extract solution was prepared (30% TBP in kerosene)
Research undertaken to determine optimum parameters pursue uranium extraction of impure uranyl nitrate solutions (AUI) with tributhylphosphate (TBP) by:
Ø determining the degree of saturation of organic phase uranium;
Ø uranium depletion in the aqueous phase and
– Reextraction degree of uranium in organic phase saturated.
CO (INCDMRR – Bucharest) with its project partners, UPB – CCEEM, UBC) realized uranium purification modeling by liquid-liquid extraction by determination in terms of purification efficiency to achieve optimum process parameters.
Dependent variable for purification operation was considered extraction yield and independent variables were considered:
Uranium content in aqueous phase;
Free acidity;
Concentration of tributhylphosphate in kerosene;
Organic – aqueous phase ration;
Number of extraction steps.
For this study was used to make a relatively large number of experiments that examined the effects of each of three variables presented in tables 1 and 2.
Table nr. 1. Considered parameters and their variable domain
| Parameter | Reduce variable | Minimal value (-1) | Medium value (0) | Maximum value (+1) |
| Uranium content in aqueous phase (g U/l) | x1 | 280 | 300 | 320 |
| A:O ratio | x2 | 1:2,8 | 1:3 | 1:3,2 |
| Number of extraction steps (steps) | x3 | 6 | 8 | 10 |
Table nr. 2. Considered parameters and their variable domain
| Parameter | Reduce variable | Minimal value (-1) | Medium value (0) | Maximum value (+1) |
| Uranium content in aqueous (g U/l) | x1 | 280 | 300 | 320 |
| Free acidity in aqueous phase (N) | x2 | 1:2,8 | 1:3 | 1:3,2 |
| Number of extraction steps (steps) | x3 | 6 | 8 | 10 |
The statistic model witch best describe the response function of optimization criteria for considered parameters presented in table 1, after we eliminate insignificant terms, is :
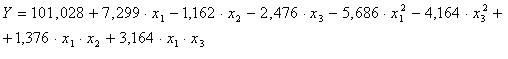
We observe that:
- Optimum content of uranium in aqueous phase is 310 g/L
- Optimum A:O ratio is 1;3;
- Optimum free acidity is 1;3,14 N;
- Optimum numbers of extraction steps is 8 steps.
In conclusion, the extraction yield tends to optimal when all variables considerate stay in the initially chosen variation range.
The statistic model witch best describe the response function of optimization criteria for considered parameters presented in table 2,after we eliminate insignificant terms, is:
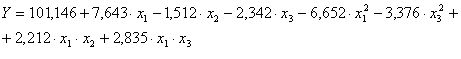
We observe that :
- Optimal content of uranium in aqueous phase is 313.6 g/L
- Optimum free acidity is 1;3,14 N;
- Optimum numbers of extraction steps is 7,88 steps.
In conclusion, the extraction yield tends to optimal when all variables considerate stay in the initially chosen variation range.
P3 (ECOPROIECT) realized a documentary study in witch treats some of theoretic and practice aspects about measurement methods of free acidity of AUI solutions at extraction/reextraction, selection and control criteria.
Was studied the form of stable emulsion by liquid-liquid extraction under the influence of lot of impurities in aqueous phase (silica content, organic substances, solid suspensions, molybdenum), making a mathematic model.
The extraction yield is dependent variable for purification operation and independent variable considered were: silica content, organic substances, solid suspension. Considered parameters and their variation domain are presented in table no. 3.
Table nr. 3. Considered parameters and their variable domain
| Parameter | Reduce variable | Minimal value (-1) | Medium value (0) | Maximum value (+1) |
| Organic substance content in aqueous phase (g/l) | x1 | 0 | 0,5 | 1 |
| Silica content in aqueous phase(g/l) | x2 | 0,05 | 0,1 | 0,15 |
| Solid suspension in aqueous phase (g/l) | x3 | 0,05 | 0,1 | 0,15 |
The statistic model witch best describe the response function of optimization criteria for considered parameters presented in table 3after we eliminate insignificant terms, is:
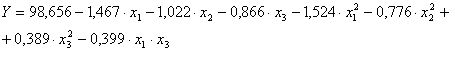
We observe that:
- Optimal content of organic substances in aqueous phase is 0.2065 g/L;
- Optimal silica content in aqueous phase is 0.06 g/L;
- Optimal solid suspensions in aqueous phase is14 g/L.
For the study about the influence of low temperature to extraction yield, was achieved a mathematical model established that independent variable are uranium content in aqueous phase, free acidity in aqueous phase, extraction and dependent variable is extraction yield, this all are presented in table no. 4:
Table nr. 4 Considered parameters and their variable domain
| Parameter | Reduce variable | Minimal value (-1) | Medium value (0) | Maximum value (+1) |
| Uranium content in aqueous phase (g U/L) | x1 | 280 | 300 | 320 |
| Free acidity in aqueous phase (N) | x2 | 1:2,8 | 1:3 | 1:3,2 |
| Temperature (0C) | x3 | 15 | 20 | 25 |
Mathematic model is that:
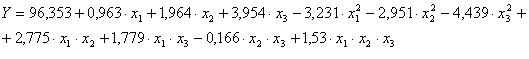
We observe that:
- Optimal content of uranium in aqueous phase is 307.14 gU/L;
- Optimal free acidity in aqueous phase is 1:3.1N;
- Optimal extraction temperature is 22.60 0C.
Project results dissemination were made :
- organization in partnership P2 (Bacau University) – CO (INCDMRR – Bucharest) – of International Applied Sciences – Chemistry and Chemical Engineering Conference – CISA 2008, 4.- 6 April 2008, Slanic Moldova, Bacau, with international participation, after witch a proceeding wad edited with quote ISBN 978 – 973- 1833-86-6.
- Work visits – good practice exchange in France – Cote d`Opale du Littoral University, Dunkerque – CO and Torino Polytechnics University – P2.
Stage III deadline – 2009 30.11
Formation of stable emulsions research subject to degradation products of the TBF’s.
Specific objectives
- Incident study of degradation of tributhylphosphate above stable emulsion;
- Molybdenum content influence study above extractant`s degradation;
- Development conceptual model of technological flow and optimal description of the automatic extraction/reextraction with organic and aqueous phase’s battery power.
Phase’s abstract
The extractant, TBS- kerosene used in uranium purification process by liquid-liquid extraction, is depredated both by radiations and nitric acid hydrolysis. [4]
TBP hydrolysis formation resulting gradually di butyl phosphoric acid, mono-butyl phosphoric acid, phosphoric acid and buthanol as reactions:
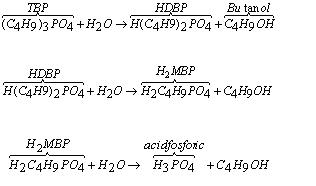
HDBP and H2MBP retain highly actinide and fission products with formation of complex combination. Must be avoided precipitation, because this will lead to the formation of third phase at the interface between the organic and aqueous phase, but also to cover bottle’s walls.
The formation of the third phase leads to important losses of uranium. Solvent (kerosene) also can contain metal ions (like molybdenum) witch were extracted alongside with uranium, this ions can accelerate TBP degradation from aqueous phase to form aromatics nitro-compounds.
Factors witch influence TBP hydrolysis are:
- HNO3 concentration;
- Uranium concentration;
- Temperature;
- Impurities from solvent.
Laboratory tests were carried out in order to establish the influence of degradation products of TBP above uranium extraction, and the obtained results showing that the process of loading in the solvent metal is not adversely affected by this breakdown products, mono and di butyl phosphate, with a good load capacity, but the presence of different metals salts (molybdenum) can form insoluble salts with degradation products, favoring the emergence of the third phase.
At uranium reextraction found that with increasing content of degradation products in solvent harder to discharge resulting in metal reextraction. At contents higher than 1% degradation products in solvent, appears obvious negative influence on the reextraction process.
Mathematical modeling was done to influence degradation products of TBF above stable emulsion formation. Dependent variable was third phase volume, and independent variable considered were:
- Content of degradation products (mono- and di butyl phosphate) from organic solvent (g/L);
- Molybdenum content from AUI (g/l)
- Aqueous phase free acidity (N).
Mathematical model is:
![]()
The third phase volume tends to a minimal value in condition:
- Degradation products content is not much that 0.58% from TBA/kerosene content;
- Molybdenum content is maximum 0.055 g/l
- Aqueous phase has a free acidity of 3.02 N.
Optimum model flow technology was achieved uranium purification by liquid-liquid extraction with optimal technological parameter presentation list for dissolution steps – maturation, filtration, conditioning and extraction.
After uranium purification modeling has came to conclusion that optimal parameters for extraction process are:
- Optimal uranium content in aqueous phase is 310-315 gU/L;
- Optimal A:O ratio is 1:3;
- Optimal free acidity in aqueous phase is 3.14 – 3.19 N;
- TBP concentration in kerosene is 30%;
- Optimal number of extraction steps is 8 steps
Results dissemination resulted:
Organization in partnership with Bacau University – INCDMRR – Bucharest – 2 of International Conference of Applied Sciences, Chemistry and Chemical engineering – CISA 2009, 2-5 april 2009 at Slanic Moldova, Bacau, with international participation, after witch a proceeding wad edited with quote ISSN 2066-7817;
Organization in partnership with Bacau University – INCDMRR- Buicharest – 20-26.08 2009 of “Summer school” in South Eforie, COnstanta;
Antoneta Filcenco- 0lteanu, Eugenia Panturu, Nicoleta Groza, Viorica Ciocan , ´Mathematical modelling of uranium purification by liquid – liquid extraction” 1st Internaional Exergy, Life Cycle Assessment and Sustainability Workshop & Symposium, ELCAS 2009 , 4-6 June 2009, Nysyros Island Greece, Workshop Proceedings
Filcenco-Olteanu, E. Panturu, V. Ciocan, N. Groza, M. Flucus, L. Grigoras “Study the influence of process variables on the efficiency of uranium extraction “ Timisoara Academic Days, “Chem. Bull. “POLITEHNICA” Univ. (Timişoara), F. 1-2-2009, p. 1036-1040, Ed. Mirton ISBN 978-973-52-0630-7,
Antoneta Filcenco- 0lteanu, Eugenia Panturu, Nicoleta Groza, Viorica Ciocan , ´Mathematical modelling of uranium purification by liquid – liquid extraction” The XIII Balkan Mineral Processing Congress, Vol. 2 , p. 544 -548, 2009, ISBN 978-973-677-161-3;
Filcenco.A, Panturu E. Ciocan .V., Groza N, Flucus, M., Grigoras L “Study the influence of process variables on the efficiency of uranium extraction” Conferintei Interanationale de stiinte Aplicate – Chimie si Inginerie chimica – CISA 2008, in perioada 4 – 6 aprilie 2008 la Slanic Moldova – jud. Bacau, ISSN 2066 – 7817.
Antoneta Filcenco- 0lteanu, Eugenia Panturu, Nicoleta Groza, Viorica Ciocan, “Waste water management from acid uranium ore processing” 42nd IUPAC Congress Chemistry solutions, 2 – 7 aug. 2009/SEECC, Glasgow, Scotland, UK, Abstract Book, Ed. RSC, p407-014;
Phase IV – Done 15.09. – 15.12.2009
Research of uranium reextraction from organic phase
Because the characteristics of chemical concentrates depend on the characteristics and performance of original hydrometallurgical process used in the refining stage is necessary to reduce the concentration of impurities of several hundreds or thousands of times.
For avoiding contamination of the final product, is necessary to wash the loaded organic phase, so that extracts impurities along with uranium not reaching the final product.
remove impurities by washing can be accomplished by two mechanisms, namely:
- Washing with acidulated water for removing the impurities. If the free acidity is higher the uranium distribution coefficient is higher too.
- Chemical removal of impurities trough a stripping mechanism, we use a pure uranyl nitrate solution. The impurities form loaded organic phase is depressed through substitution of uranyl from purified uranium aqueous phase.
Has developed a statistical model describing the influence of washing conditions on process efficiency, as independent variables use the phase’s ratio, acidity and number of steps where washing was done.
The statistical model witch best describes the response function of optimal criteria is:
![]()
The third phase volume tends to a minimal value in condition:
- Degradation products content is not much that 0.58% from TBA/kerosene content;
- Molybdenum content is maximum 0.055 g/l
- Aqueous phase has a free acidity of 3.02 N.
Optimum model flow technology was achieved uranium purification by liquid-liquid extraction with optimal technological parameter presentation list for dissolution steps – maturation, filtration, conditioning and extraction.
After uranium purification modeling has came to conclusion that optimal parameters for extraction process are:
- Optimal uranium content in aqueous phase is 310-315 gU/L;
- Optimal A:O ratio is 1:3;
- Optimal free acidity in aqueous phase is 3.14 – 3.19 N;
- TBP concentration in kerosene is 30%;
- Optimal number of extraction steps is 8 steps
Results dissemination resulted:
Organization in partnership with Bacau University – INCDMRR – Bucharest – 2 of International Conference of Applied Sciences, Chemistry and Chemical engineering – CISA 2009, 2-5 april 2009 at Slanic Moldova, Bacau, with international participation, after witch a proceeding wad edited with quote ISSN 2066-7817;
Organization in partnership with Bacau University – INCDMRR- Buicharest – 20-26.08 2009 of “Summer school” in South Eforie, COnstanta;
Antoneta Filcenco- 0lteanu, Eugenia Panturu, Nicoleta Groza, Viorica Ciocan , ´Mathematical modelling of uranium purification by liquid – liquid extraction” 1st Internaional Exergy, Life Cycle Assessment and Sustainability Workshop & Symposium, ELCAS 2009 , 4-6 June 2009, Nysyros Island Greece, Workshop Proceedings
Filcenco-Olteanu, E. Panturu, V. Ciocan, N. Groza, M. Flucus, L. Grigoras “Study the influence of process variables on the efficiency of uranium extraction “ Timisoara Academic Days, “Chem. Bull. “POLITEHNICA” Univ. (Timişoara), F. 1-2-2009, p. 1036-1040, Ed. Mirton ISBN 978-973-52-0630-7,
Antoneta Filcenco- 0lteanu, Eugenia Panturu, Nicoleta Groza, Viorica Ciocan , ´Mathematical modelling of uranium purification by liquid – liquid extraction” The XIII Balkan Mineral Processing Congress, Vol. 2 , p. 544 -548, 2009, ISBN 978-973-677-161-3;
Filcenco.A, Panturu E. Ciocan .V., Groza N, Flucus, M., Grigoras L “Study the influence of process variables on the efficiency of uranium extraction” Conferintei Interanationale de stiinte Aplicate – Chimie si Inginerie chimica – CISA 2008, in perioada 4 – 6 aprilie 2008 la Slanic Moldova – jud. Bacau, ISSN 2066 – 7817.
Antoneta Filcenco- 0lteanu, Eugenia Panturu, Nicoleta Groza, Viorica Ciocan, “Waste water management from acid uranium ore processing” 42nd IUPAC Congress Chemistry solutions, 2 – 7 aug. 2009/SEECC, Glasgow, Scotland, UK, Abstract Book, Ed. RSC, p407-014;
Phase IV – Done 15.09. – 15.12.2009
Research of uranium reextraction from organic phase
Because the characteristics of chemical concentrates depend on the characteristics and performance of original hydrometallurgical process used in the refining stage is necessary to reduce the concentration of impurities of several hundreds or thousands of times.
For avoiding contamination of the final product, is necessary to wash the loaded organic phase, so that extracts impurities along with uranium not reaching the final product.
remove impurities by washing can be accomplished by two mechanisms, namely:
- Washing with acidulated water for removing the impurities. If the free acidity is higher the uranium distribution coefficient is higher too.
- Chemical removal of impurities trough a stripping mechanism, we use a pure uranyl nitrate solution. The impurities form loaded organic phase is depressed through substitution of uranyl from purified uranium aqueous phase.
Has developed a statistical model describing the influence of washing conditions on process efficiency, as independent variables use the phase’s ratio, acidity and number of steps where washing was done.
The statistical model witch best describes the response function of optimal criteria is:
![]()
Statistical analysis of the model for washing the organic phase loaded with uranium indicates that we have a good correlation between experimental values from measurements and calculated values, the residual error is smaller.
Optimum model flow technology was achieved uranium purification by liquid-liquid extraction with optimal technological parameter presentation list for dissolution steps – maturation, filtration, conditioning and extraction, loaded solvent wash and reextraction.
Results dissemination was realized through presentation and publication of abstract : Study the influence of process variables of efficiency of uranium extraction: , authors A. Filcenco – 0lteanu, E.Panturu, V.Ciocan, N.Groza, L.Grigoras, la 16th Romanian International Conference on Chemistry and Chemical Engineering –RICCCE sept. 2009 , pag. SIV 186 , Ed. Printech Bucuresti , ISBN 978-606-521-349-4.
Stage IV – done
Applied research, technological development and support activities
- Specifics objectives
- Operating manual preparation;
- Demonstration of viability developed technological flow
- Result disemination
Optimum purification process uranium, the only industrial application, is liquid-liquid extraction, using as extractant solution Tributyl-n-phosphate (TBF) in kerosene. Purification of uranium extraction from aqueous phase to organic phase is achieved by selective transfer of UO2(NO3)2 aqueous phase organic phase as the TBF solvation complex, UO2(NO3)2∙2TBF, while most other nitrogen Inorganic remain in aqueous phase. The level of impurities in uranium aqueous phase, degradation of TBF’s (mono and dibutyl phosphate), low acidity of the aqueous solution (<2.8 N HNO3) and low temperature extraction environment favors the appearance of stable emulsions (the third phase) at the interface aqueous and organic phases. Modernized technology developed under this project is based on limiting the losses of uranium purification operation by reducing the incidence of factors which lead to the formation of stable emulsions by: – technical use of uranium concentrate to the specification of product to achieve impure uranyl nitrate solution;
Ø filtering impure solution of uranyl nitrate on a suitable filter material to a temperature which not exceeding 30-35 0C to obtain a clear solution, free of solids;
Ø precise temperature control during winding operations, maturation, filtration;
Ø the use of impure uranyl nitrate solution without organic substances in the uranium extraction stage;
Ø control and correction of HNO3 acid during extraction operation as a low aqueous phase acidity of 2.8 N HNO3, lead precipitates which are stable emulsions forming seeds;
Ø heat extraction medium during periods of low temperatures above 200C to avoid the appearance of crystals of UO2 (NO3)2 during the extraction process;
Ø washing solvent after extraction – reextraction to prevent quality. depreciation
Application of the proposed technology will lead to:
Ø reducing losses of uranium in uranium purification operation by reducing the incidence of factors which lead to the formation of stable emulsions
Ø increasing the efficiency of purifying uranium liquid-liquid extraction operation with 5%
Ø reducing production costs per unit of product
Ø opportunities for technology transfer, without major investment
Results dissemination resulted:
Ø Organization in partnership with Bacau University – INCDMRR – Bucharest – of International Conference of Applied Sciences, Chemistry and Chemical engineering – CISA 2010, 8-11april , at Slanic Moldova, Bacau, with international participation, after witch a proceeding wad edited with quote ISSN 2066-7817;
Ø Roundtable at International Conference of Applied Sciences, Chemistry and Chemical Engineering – CISA 2010, 8-11of April , at Slanic Moldova, Bacau,
Ø papers presented at scientific meetings and published in journals ISBN – ISSN:
- Statistical modeling of uranium purification by liquid-liquid extraction. A. Filcenco-Olteanu, E. Panturu, L. Grigoras, R.-I. Panturu –7th European Congress of Chemical and Process Engineering Praga, CHISA, 28 aug.-2 sept. 2010 – , ISBN 978-80-02-02250-3
- Some Theoretical Aspects on the Third Phase Formation in Uranium Solvent Extraction, A. Filcenco – Olteanu, E. Panturu, Gh. Filip, L. Grigoras, Conferinta Internationala de Stiinte Aplicate, Chimie si Inginerie Chimica-CISA 2010, Bacau-Slanic Moldova, 8 – 11 aprilie 2010, p. 388-394, ISSN 2066-7817 , Ed. Alma Mater, Bacau;
- Uranium Solvent Extraction using ultrasound, E. Panturu, A. Filcenco-Olteanu, Nicoleta Groza Conferinta Internationala de Stiinte Aplicate, Chimie si Inginerie Chimica-CISA 2010, Bacau-Slanic Moldova, 8 – 11 aprilie 2010, p. 404-409, ISSN 2066-7817 , Ed. Alma Mater, Bacau
- Statistical study of factors affecting the uranium extraction with TBP in Kerosene A. Filenco – Olteanu, E. Panturu, L. Grigoras, N. Groza, European Nuclear Conference, Barcelona, Spania, 30.mai – 2 iunie, 2010, aceptat spre publicare, ISBN 978-92-95064-09-6

